Pakistan approves Chinese Sinopharm COVID-19 vaccine for emergency use
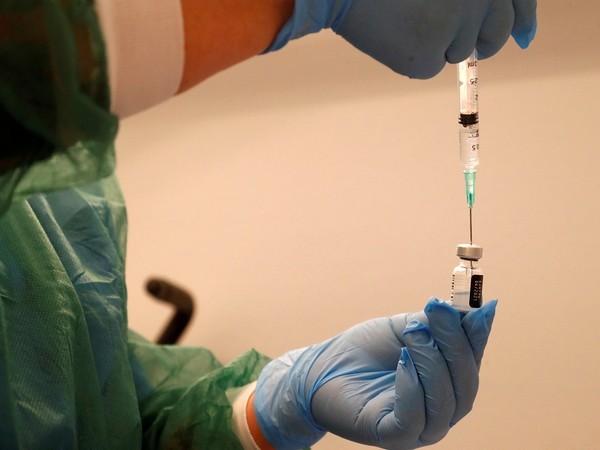
Pakistan on Monday approved the Chinese Sinopharm COVID-19 vaccine for emergency use, a government statement said. Pakistan's health ministry has said the country was in process of speaking to different vaccine candidates.
- Country:
- Pakistan
Pakistan on Monday approved the Chinese Sinopharm COVID-19 vaccine for emergency use, a government statement said. The Drug Regulatory Authority Pakistan (DRAP) said the vaccine, manufactured by China National Pharmaceutical Group (SinoPharm), had been given emergency use approval (EUA).
The authority had approved AstraZeneca's vaccine, developed with Oxford University, for emergency use at the weekend. Pakistan's health ministry has said the country was in process of speaking to different vaccine candidates.
(This story has not been edited by Devdiscourse staff and is auto-generated from a syndicated feed.)
- READ MORE ON:
- AstraZeneca
- Chinese
- Pakistan
- Oxford University
- SinoPharm
Advertisement
ALSO READ
China commerce minister kickstarts Europe trip with Chinese EV firms meeting
Rain and Chinese demand to boost Australia's wheat, barley planting
Yellen says U.S. will not accept new industries being decimated by cheap Chinese imports
Yellen says US will not accept Chinese imports decimating new industries
Sweden expels a Chinese journalist, calling her a threat to national security, report says










