Breakthrough AI system exposes silent diabetes threats in real time
Several machine learning models were evaluated, including Logistic Regression, XGBoost, Random Forest, and Neural Networks, across three sampling strategies: original distribution, SMOTE oversampling, and random undersampling. LightGBM paired with random undersampling consistently outperformed others in recall - a critical metric in healthcare to minimize false negatives - making it the final model deployed in the application.
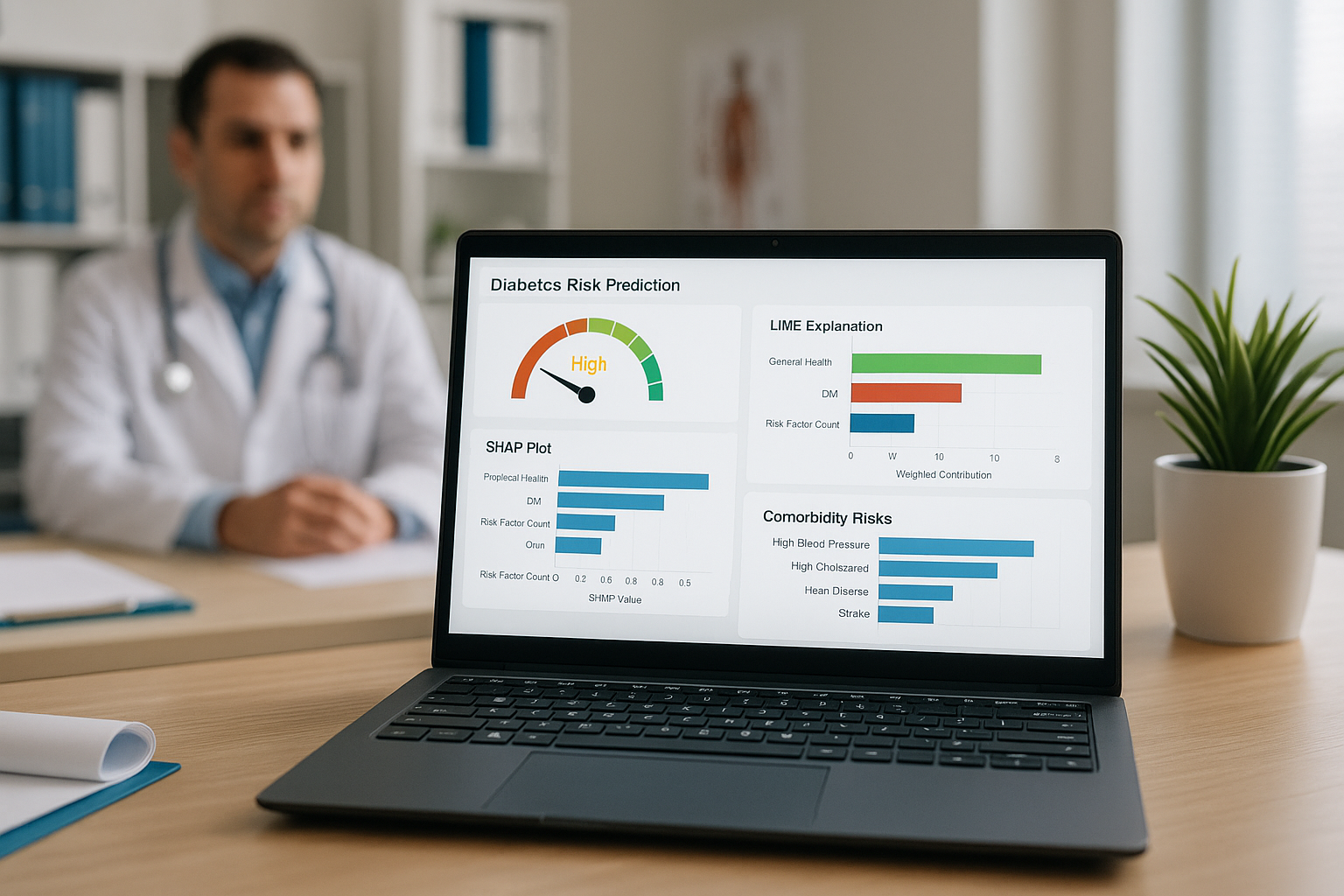
A new study has unveiled a machine learning-driven web application capable of predicting diabetes risk and delivering interpretable results in real time. The study, titled “Interactive Diabetes Risk Prediction Using Explainable Machine Learning: A Dash-Based Approach with SHAP, LIME, and Comorbidity Insights” and published on arXiv, introduces a LightGBM-based classification model integrated with explainable AI techniques and deployed in a user-friendly web interface to support both clinical screening and public awareness campaigns.
The system analyzes behavioral, demographic, and clinical features from the Behavioral Risk Factor Surveillance System (BRFSS) dataset to provide users with immediate risk scores, contributing factors, and comorbidity alerts - all accessible through a browser-based interface. The tool aims to bridge the gap between complex AI systems and patient-centric preventive healthcare.
How was the AI system developed and what makes it different?
The model was trained using over 250,000 records from the 2015 BRFSS dataset, a large-scale health-related survey conducted by the U.S. Centers for Disease Control and Prevention. The study focused exclusively on binary classification, diabetic versus non-diabetic, by removing prediabetic entries to simplify predictive clarity.
Several machine learning models were evaluated, including Logistic Regression, XGBoost, Random Forest, and Neural Networks, across three sampling strategies: original distribution, SMOTE oversampling, and random undersampling. LightGBM paired with random undersampling consistently outperformed others in recall - a critical metric in healthcare to minimize false negatives - making it the final model deployed in the application.
To address transparency and usability, the study incorporated both SHAP (SHapley Additive exPlanations) and LIME (Local Interpretable Model-Agnostic Explanations). These techniques helped visualize which features most influenced predictions, both globally across the dataset and locally for individual users. Additionally, engineered features like a lifestyle score, healthcare access score, and cumulative risk factor count improved interpretability and decision-making.
What sets this system apart is its deployment: rather than remaining a static academic model, it was converted into a live, multi-step interactive web application using the Dash framework. This application collects lifestyle, medical, and socioeconomic data from users and returns a real-time risk analysis, supported by SHAP plots, LIME visualizations, and comorbidity alerts.
What Did the Results Show About Performance and Accuracy?
Across multiple validation scenarios, LightGBM emerged as the top-performing model when paired with a random undersampling strategy. The model achieved superior recall while maintaining competitive accuracy and F1-scores, making it well-suited for healthcare environments where early detection is essential.
A statistical analysis using one-way ANOVA and Tukey’s HSD post-hoc test confirmed that the performance differences between LightGBM and competing models were statistically significant, especially under undersampling. These tests validated the model's superiority not just in raw metrics but also in consistency across multiple folds and data samples.
SHAP-based global interpretability showed that the most influential predictors were:
- Risk Factor Count: A sum of chronic conditions such as high blood pressure, high cholesterol, stroke, and heart disease.
- General Health and BMI: Indicators of overall wellness.
- Difficulty Walking and Cholesterol Check Frequency: Reflecting functional health and preventive care engagement.
At the individual level, SHAP waterfall plots and LIME bar charts revealed specific variables driving predictions. For instance, a combination of high BMI, poor physical health days, and chronic conditions led to high-risk predictions, with visual breakdowns helping users understand how and why these results were generated.
These interpretability tools not only increase transparency but also provide actionable insights. Users can identify which modifiable behaviors, such as smoking or physical activity, contribute to their risk and receive lifestyle recommendations accordingly.
How Does the Application Support Public Health and Future Expansion?
Beyond the model’s accuracy and interpretability, the application is designed for widespread usability and accessibility. The interface follows a five-step form: basic information, lifestyle habits, medical history, healthcare access, and socioeconomic data. Upon submission, users receive a risk prediction, explanatory visuals, and recommendations to mitigate identified risks.
Crucially, the app also surfaces comorbidity insights by calculating Pearson correlations between diabetes and related chronic conditions. The study found the strongest associations with high blood pressure (r = 0.26), high cholesterol (r = 0.20), heart disease (r = 0.17), and stroke (r = 0.10). These correlations are flagged for users, encouraging proactive monitoring of overlapping health risks.
From a public health perspective, this application serves multiple roles:
- Self-screening tool for individuals without regular healthcare access.
- Support tool for clinicians seeking interpretable risk assessments.
- Educational platform for raising awareness of modifiable risk factors.
Looking ahead, the research outlines several areas for enhancement. These include expanding beyond diabetes to predict multiple chronic conditions, integrating longitudinal or real-time wearable data, adding support for multiple languages, and validating the system with clinical partners. The modular design also supports future integration with electronic health records and feedback loops for continual model refinement.
- FIRST PUBLISHED IN:
- Devdiscourse










