Anti-viral drug favipiravir gets DGCI nod for 'restricted emergency use' in mild to moderate COVID-19 cases
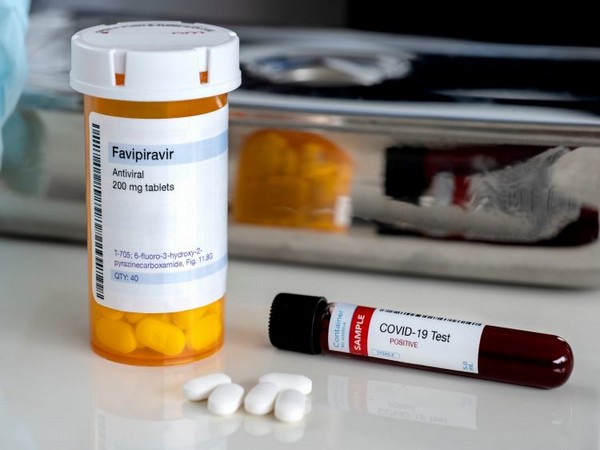
The Drug Controller General of India (DCGI) has granted permission to anti-viral drug favipiravir for "restricted emergency use" in mild to moderate cases of COVID-19, a senior government official confirmed to ANI.
- Country:
- India
The Drug Controller General of India (DCGI) has granted permission to anti-viral drug favipiravir for "restricted emergency use" in mild to moderate cases of COVID-19, a senior government official confirmed to ANI.
The country's top drug regulator has given a fast-tracked permission to an indigenous pharmaceutical company -- Glenmark to manufacture and market favipiravir (200 mg) tablet. This approval has been granted based on the evaluation of data and in consultation with the Subject Expert Committee, as part of the accelerated approval process, considering the emergency situation and unmet medical need of the COVID-19 outbreak. It is for restricted emergency use in India.
Restricted use entails responsible medication use where every patient must have signed informed consent before treatment initiation. A senior health ministry official told ANI, "The DCGI has approved favipiravir drug for restricted emergency use for the treatment of mild to moderate cases of COVID-19. This emergency use authorisation has been given on several conditions. The company has to take written consent from each patient. Also, in the first 1,000 patients, the pharma company has to do active post-market surveillance to evaluate the efficacy and safety of the drug being administered."
"The patient will receive a dose of 3,600 mg on the first day as a loading dose. After this, the patient would receive 1,800 mg for some days which will depend upon his/her viral load," he said."The drug cannot be given to patients with severe liver and renal diseases including pregnant and lactating mother," added the official. Now that permission has been granted and the company has to get the license to manufacture from the Central Drugs Standard Control Organisation (CDSCO) under the Drug and Cosmetic Act and Rules.
(This story has not been edited by Devdiscourse staff and is auto-generated from a syndicated feed.)
- READ MORE ON:
- Glenmark
- Central Drugs Standard Control Organisation










