Health News Roundup: FDA could expand remdesivir use despite mixed data; WHO chief hopes coronavirus pandemic will last less than two years and more
Exclusive: Nearly a fifth of enrollees in Pfizer, BioNTech COVID-19 vaccine study are Black or Latino Nearly a fifth of 11,000 people enrolled so far in a 30,000-volunteer U.S. trial testing a COVID-19 vaccine from Pfizer and German partner BioNTech are Black or Latino, groups among the hardest hit by the coronavirus virus pandemic, a top Pfizer executive said.
Following is a summary of current health news briefs.
U.S. CDC reports 173,490 deaths from coronavirus
The U.S. Centers for Disease Control and Prevention (CDC) on Friday said the number of deaths due to the new coronavirus had risen by 1,074 to 173,490 and reported 5,551,793 cases, an increase of 44,864 cases from its previous count. The CDC reported its tally of cases of the respiratory illness known as COVID-19, caused by a new coronavirus, as of 4 pm ET on Aug. 20 versus its previous report a day earlier.
COVID-19 era highlights U.S. 'black hole' compensation fund for pandemic vaccine injuries
A U.S. government program that compensates people who say they have been harmed by an emergency vaccine has paid out on fewer than 10% of claims, raising questions whether the process should be used to address any potential side effects from a coronavirus shot, according to some lawyers who have filed such claims. The Countermeasures Injury Compensation Program (CICP), run by an agency under the U.S. Department of Health and Human Services (HHS), has been designated to handle any issues with a COVID-19 vaccine.
What you need to know about the coronavirus right now
Here's what you need to know about the coronavirus right now: New, milder, virus variant found Australia coronavirus hotspot state records 13 new deaths, stable infections
Australia's second most populous state, Victoria, reported 13 new coronavirus deaths in the 24 hours to Saturday morning, authorities said, while new infections in the hotspot state remained below 200 for the second consecutive day. Other than in Victoria, which accounts for over 80% of the country's COVID-19 deaths due to a second wave of infections, Australia has large avoided the high casualty numbers of many nations with just under 24,500 infections and 485 deaths.
Moderna says more than 40% of participants enrolled for COVID-19 vaccine trial
Drug developer Moderna Inc on Friday said it has so far enrolled 13,194 participants in the ongoing late-stage 30,000-volunteer U.S. trial testing its COVID-19 vaccine candidate. In a tweet, the company also said that 18% of the participants currently enrolled are Black, Latino, American Indian or Alaska Native, groups among the hardest hit by the coronavirus virus pandemic.
Limited transmission of COVID-19 found in U.S. childcare study, CDC says
Transmission of COVID-19 from children or adults to other people in Rhode Island childcare programs occurred on only a limited basis, a study by the U.S. Centers for Disease Control and Prevention showed on Friday. CDC Director Robert Redfield told reporters on a call that the findings indicated that there is a path "to get these childcare programs to reopen, which are very important for our country."
WHO chief hopes coronavirus pandemic will last less than two years
The World Health Organization hopes the coronavirus pandemic will be shorter than the 1918 Spanish flu and last less than two years, WHO chief Tedros Adhanom Ghebreyesus said on Friday if the world unites and succeeds in finding a vaccine. The WHO has always been cautious about giving estimates on how quickly the pandemic can be dealt with while there is no proven vaccine.
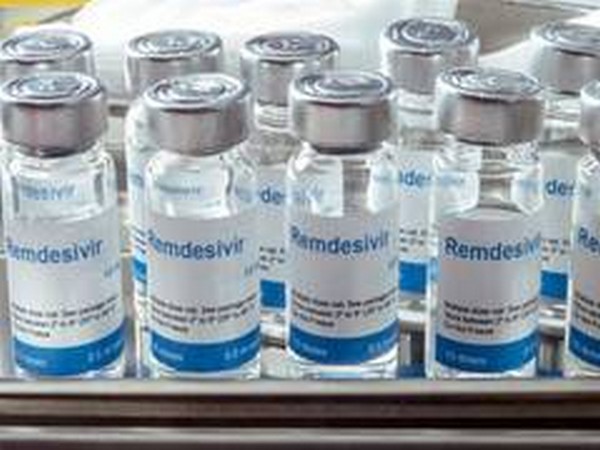
Gilead: FDA could expand remdesivir use despite mixed data
The U.S. Food and Drug Administration could update its emergency use authorization for Gilead Sciences Inc's drug remdesivir to include patients hospitalized with moderate COVID-19, despite mixed trial results, the company's top research executive said on Friday. The FDA in May okayed sales of remdesivir on an emergency basis for patients hospitalized with severe COVID-19, the disease caused by the new coronavirus, after trial data showed that the antiviral drug helped shorten their hospital recovery time.
Explainer: Reaching herd immunity in a viral pandemic
The novel coronavirus pandemic has brought "herd immunity" to the public consciousness, kindling hope the phenomenon can help slow or even end the outbreak. Herd immunity refers to a large portion of a community developing a degree of immunity to a virus, thereby reducing person-to-person spread. As a result, the whole community gains protection, not just those who are immune.
Exclusive: Nearly a fifth of enrollees in Pfizer, BioNTech COVID-19 vaccine study are Black or Latino
Nearly a fifth of 11,000 people enrolled so far in a 30,000-volunteer U.S. trial testing a COVID-19 vaccine from Pfizer and German partner BioNTech are Black or Latino, groups among the hardest hit by the coronavirus virus pandemic, a top Pfizer executive said. "Between Latinx and Black or African American populations, we're running at about 19 percent or so," Dr. Bill Gruber, Pfizer’s senior vice president of vaccine clinical research and development, told Reuters in an interview.
(With inputs from agencies.)
ALSO READ
Miracle Survival Amidst Tragedy: Girl Survives Deadly Spanish Train Crash
Spanish Rail Safety Concerns Surge
EXCLUSIVE-Investigators find broken joint on track at Spanish rail crash site, source says
Mbappé defends Vinícius after boos by Real Madrid fans in Spanish league match
Death toll in Spanish train collision rises to 40 as authorities fear more bodies could be found










